How Viral Infections Can Trigger Hashimoto's — And Inform New Treatments

What triggered your Hashimoto's? The answer is different for everyone — and can even involve multiple causes. But one of those causes could be a viral infection.
According to a 2023 study from two medial universities in Korea, "several lines of evidence suggested that viral infection and the host immune response are affected during [Hashimoto's Thyroiditis]. The researchers came to this conclusion after using a bioinformatics and connectivity map analysis.
- Genetic disposition. Some people have different versions of the gene that codes for immune cells that distinguish "foreign" from "self." And these versions of the gene are such that their immune cells are less likely to correctly make that distinction when it comes to Hashimoto's.
- Molecular mimicry. Viruses and the body have similar shapes, so the body attacks itself in an attempt to attack the virus. But as the body destroys tissue, it creates even more antigens that drive the immune (now autoimmune) response into overdrive.
- Case studies. Certain case studies showed that common viruses like herpes simplex, Epstein-Barr, and Hepatitis C were associated with onset of Hashimoto's.
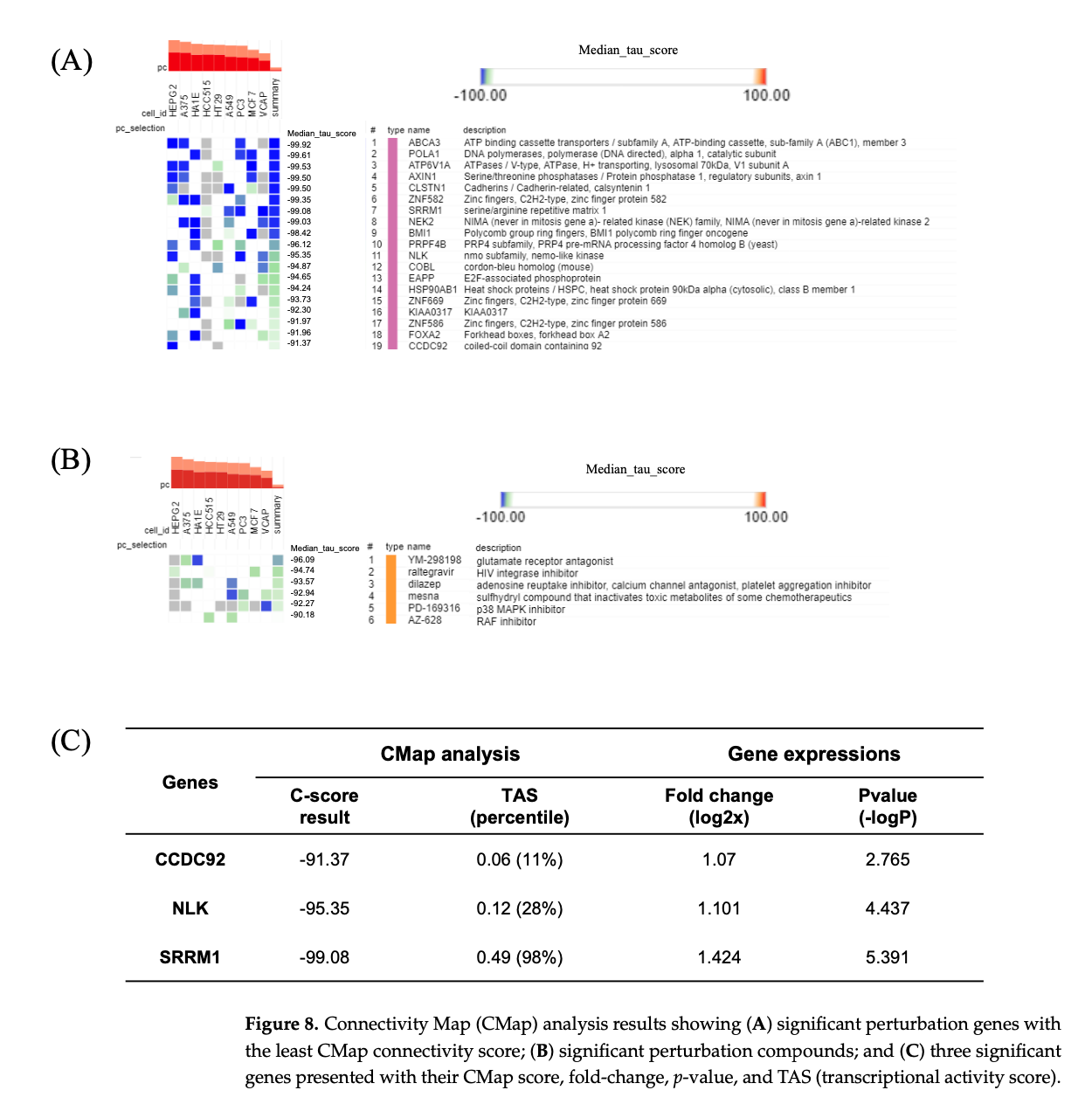
- Databases and analytics. The researchers were able to use huge, growing genetic databases – including the Connectivity Map – to get their information. Then they used an advanced analytical method (GSEA) to make sense of the data and find these insights.
- Therapeutic potential. After identifying genes associated with the onset of Hashimoto's, the researchers also identified compounds that could change the expression of those genes so that they would stop coding for things that were putting the body in an autoimmune state.
All in all, identifying how viruses influence the start of Hashimoto's can lead to new treatments that target those genes and their expression. This research is a pretty long way off, but it's important and encouraging to better understand the disease, and know there are potential new therapies that target the actual autoimmune process.
To learn more about the details of the study, read on.
Genetic Disposition
Genetic predisposition and expression changes influence the autoimmune thyroid disease course by affecting host immunoreactivity or antigen presentation/recognition [12]. Polymorphisms in IL2 and CTLA4 or the upregulation of MHC Class II molecules are impli- cated in the etiology of AID [10]. MHC, which is also known as human leukocyte antigen (HLA) in humans, plays a key role in AID by helping the immune system distinguish self from foreign, owing to its unlimited allelic diversity [13]. HLA alleles (HLA-DPB1) and their variants (HLA-DPB1*02:02 and HLA-DPB1*05:01) have been described as contributors to the early pathogenesis of autoimmune thyroiditis [14]. However, genetic susceptibility alone is often insufficient to give rise to autoimmunity [15].
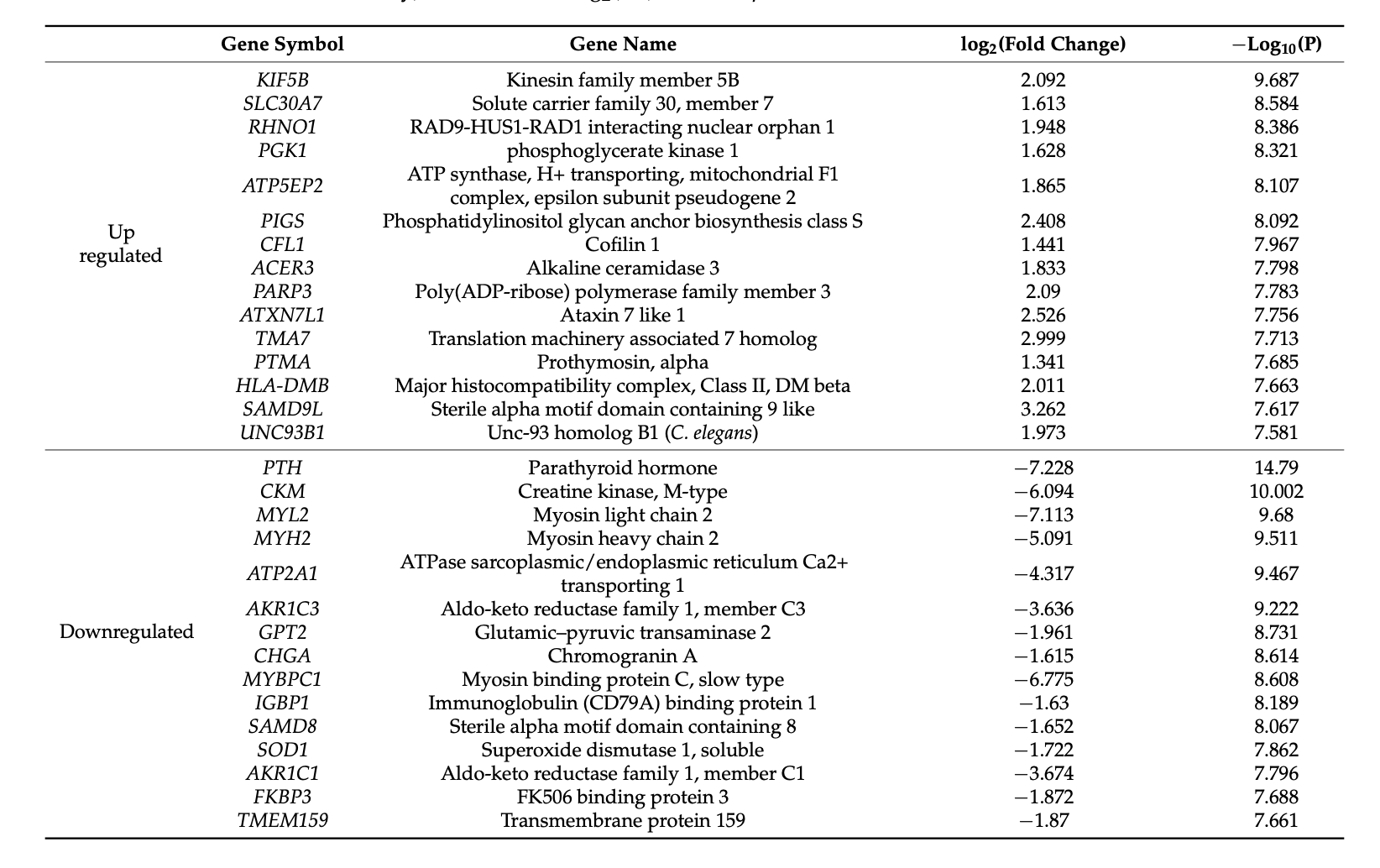
The Human Leukocyte Antigen (HLA) is plays a role in helping the body "distinguish self from foreign." However, some people with Hashimoto's have a version of this gene that isn't as good at the "distinguishing" job.
In fact, studies have shown that up-regulated and different versions of the HLA genes like, HLA-DPB1, HLA-DPB1*02:02, and HLA-DPB1*05:01 have been identified as being part of the early development of Hashimoto's. And variations in genes like L2 and CTLA4 can also play a role.
Molecular Mimicry
Recently, viral infection has emerged as an attractive environmental trigger of autoim- munity, with multiple mechanisms described for different AIDs [16]. Molecular mimicry, bystander activation, and epitope spreading are the three major mechanisms underlying virus-induced autoimmunity [17]. Molecular mimicry is based on cross-reactivity due to the structural similarity between pathogen (viral particles) and self (self-antigens), providing a basis for virus-induced autoimmunity [18]. Subsequent tissue damage results in the release of damage-associated molecular patterns (DAMPs) that activate TLRs, leading to amplified immune activation [19]. Likewise, biomolecule-based observation has been indicating viral infections as a key factor in the induction and development of autoimmune diseases.
Basically, pathogens like viral particles and antigens in one's own body can appear similar. So then the body may attack itself, thinking it's the virus. But as it attacks the body, it creates "debris" that then signals the immune system to target to into even higher overdrive through other mechanisms.
Case Studies
A case study reported HT onset after herpes simplex virus infection (3 to 6 months) in three patients, as indicated by the presence of IgM and IgG antibodies against the virus [20]. A study of 42 HT patients revealed a high prevalence of Epstein–Barr virus infection in patient tissue (n = 42) [21]. A larger study showed a clear association of hepatitis C virus (HCV) infection and thyroid autoimmunity [22]. A systematic review of 12 studies compared epidemiological differences in thyroid dysfunction between HCV-infected and non-HCV-infected patients, indicating an increased risk of thyroid dysfunction in the former [23].
Important examples included associations between
- Herpes simplex virus
- Epstein-Barr virus
- Hepatitis C virus
and development of Hashimoto's.
Advances in Bioinformatics = Better Clues
Recent advances in microarray profiling and bioinformatics tools have enabled the analysis of massive transcriptome data, providing insight into the expression changes observed during various diseases. The Gene Expression Omnibus (GEO) database is a public repository with microarray, next-generation sequencing (NGS), and other genomics data that provides access to large datasets submitted by various researchers [24]. Gene Set Enrichment Analysis (GSEA) is a powerful analytical method used for interpreting gene-expression data via Gene Ontology (GO) terms or other gene-set collections [25]. The Connectivity Map (CMap), a comprehensive, large-scale perturbation database containing 1.5 million gene expression profiles from cultured human cells, can be used to identify potential therapeutic targets or drugs for the submitted gene signature [26]. These bioinformatics tools can be utilized to address various biomedical issues by deciphering information hidden in a large number of biological datasets [27].
The researchers were able to use huge, growing genetic databases – including the Connectivity Map – to get their information. Then they used an advanced analytical method (GSEA) to make sense of the data and find these insights.
Implications for Future Treatments
An investigation into the biomarkers of HT should be conducted to improve diagnosis and provide feasible medical treatment. A previous study on HT utilized the GEO microar- ray database yet only suggested hub genes deduced from protein–protein interaction (PPI) network of DEGs [28]. In this work, we extracted a list of DEGs from the GEO microarray database and divided them into functional clusters via PPI network construction and per- formed an overrepresentation analysis (ORA). Based on a ranked gene list, we conducted GSEA to scrutinize the skewed distribution of genes related to specific BP terms and KEGG
Since they identified the biomarkers of Hashimoto's, this can contribute to
- Improved diagnosis because they have more comprehensive, accurate biomarkers of the disease.
- Potential treatments based on targeting the mechanisms that can trigger autoimmune responses, such as molecular mimicry and up-regulated/variations of Human Leucocyte Antigen.
Conclusion
This study did a lot of work, congregating a lot of data and making important connections. These connections include identifying how viruses can change gene expression that can lead to autoimmunity and Hashimoto's.
The study also identified compounds that could potentially stop or reverse these genetic changes so that the autoimmune state triggered by a virus could be stopped. Since most treatments today don't focus on the autoimmune aspect of the disease – mainly hormone replacement and lifestyle changes – insights like these can lead to important advancements and relief for Hashimoto's patients.



